Enzymes are biological catalysts that speed up a chemical reaction by providing an alternative pathway with a lower activation energy. One enzymatic property is that they are specific relative to the reactions they catalyze. Specificity can be classed into four types. Firstly, Absolute specificity occurs when the enzyme will catalyze a single reaction. Group specificity takes place when the enzyme will act only on molecules that have fixed functional groups, such as phosphate, amino and methyl groups. Linkage specificity is induced when the enzyme will act on a certain type of chemical bond disregarding of the rest of the structure. Lastly, Stereochemical specificity is when the enzyme will react on a specific steric or optical isomer.
Enzymes do not always follow a smooth pattern of operation. Enzyme inhibitors are molecules that interact with the enzyme to prevent it from working in its usual order. The types of inhibitors include: nonspecific, irreversible, reversible.
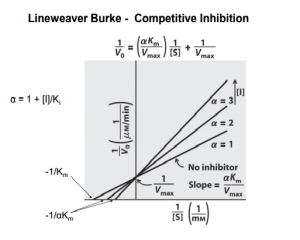
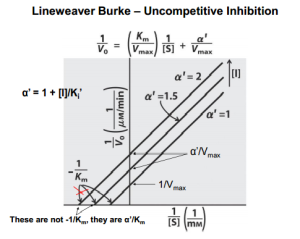
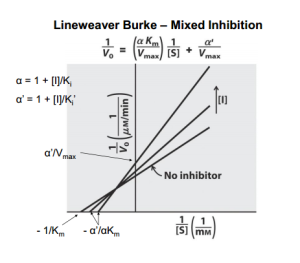
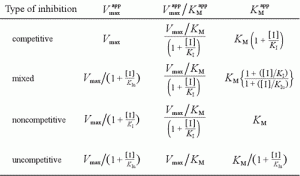
Reversible inhibition is categorized according to the effect of varying the concentration of the enzyme’s substrate on the inhibitor.
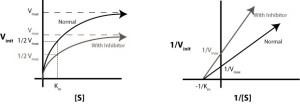
- In competitive inhibition, the inhibitor and the substrate resemble each other, and therefore cannot bind to the enzyme at the same time. The inhibitor binds to the active site of the enzyme, thus slowing the rate of reaction. The apparent Km will increase because it takes a higher concentration of the substrate to reach the Km point or half the Vmax. A competitive inhibitor will increase the apparent KM value for its substrate with no change in the apparent Vmax value.
- In uncompetitive inhibition, the inhibitor does not resemble the substrate in this type of inhibition. The inhibitor binds only to the substrate-enzyme complex. This type of inhibition causes Km and Vmax to decrease.
- In mixed inhibition, the inhibitor can bind to the enzyme at similar times as the enzyme’s substrate. The binding of the substrate and vice versa is affected by the binding of the inhibitor. This type of inhibition generally results from an allosteric effect where the inhibitor binds to a different site on an enzyme. In this type of inhibition, Vmax decreases, while Km may increase or decrease.
- Non-competitive inhibition is a form of mixed inhibition where the but does not affect the binding of substrate. The inhibitor does not resemble the substrate. Vmax will decrease due to the inability for the reaction to proceed as efficiently, but Km will remain the same as the actual binding of the substrate, by definition, will still function properly.
Lineweaver–Burk plots displaying the 4 types of reversible inhibition.