Our second quiz was given on the 6th February on Carbohydrates 1 & 2. To my surprise, along with the rest of the class, we were tested through 24 multiple choice questions and 1 “tricky” fill in the blank question. While this quiz was a little more challenging, my group and I were able to answer the questions to the best of our ability and we await the results of our painful nights studying. Fingers crossed!
Monthly Archives: February 2013
Reflection on Carbohydrates.
On Tuesday 29th January, we began to explore the exciting world of (CH2O)n. The biosignificance of Carbohydrates is expressed through it’s structural ability, storage, precursor molecules and energy. Below is a compilation of information from class lectures and online readings. Hope this simplifies any unclarity for this part of Carbohydrates! 🙂 This summary is also useful for our tutorial quizzes!
Starch and cellulose are two common carbohydrates. Both are macromolecules with molecular weights in the hundreds of thousands. Both are polymers (hence “polysaccharides”); that is, each is built from repeating units, monomers, much as a chain is built from its links. The monomers of both starch and cellulose are the same: units of the sugar glucose.
Sugars
Monosaccharides
Three common sugars share the same molecular formula: C6H12O6. Because of their six carbon atoms, each is a hexose.
They are:
- glucose, “blood sugar”, the immediate source of energy for cellular respiration
- galactose, a sugar in milk (and yogurt), and
- fructose, a sugar found in honey.
Although all three share the same molecular formula (C6H12O6), the arrangement of atoms differs in each case. Substances such as these three, which have identical molecular formulas but different structural formulas, are known as structural isomers.
Glucose, galactose, and fructose are “single” sugars or monosaccharides. Two monosaccharides can be linked together to form a “double” sugar or disaccharide.
Disaccharides
Three common disaccharides:
- sucrose — common table sugar = glucose + fructose
- lactose — major sugar in milk = glucose + galactose
- maltose — product of starch digestion = glucose + glucose
Although the process of linking the two monomers is rather complex, the end result in each case is the loss of a hydrogen atom (H) from one of the monosaccharides and a hydroxyl group (OH) from the other. The resulting linkage between the sugars is called a glycosidic bond. The molecular formula of each of these disaccharides is
C12H22O11 = 2 C6H12O6 − H2O
All sugars are very soluble in water because of their many hydroxyl groups. Although not as concentrated a fuel as fats, sugars are the most important source of energy for many cells.
Carbohydrates provide the bulk of the calories (4 kcal/gram) in most diets, and starches provide the bulk of that. Starches are polysaccharides.
Polysaccharides
Starches
Starches are polymers of glucose. Two types are found:
- Amylose consists of linear, unbranched chains of several hundred glucose residues (units). The glucose residues are linked by a glycosidic bond between their #1 and #4 carbon atoms.
- Amylopectin differs from amylose in being highly branched. At approximately every thirtieth residue along the chain, a short side chain is attached by a glycosidic bond to the #6 carbon atom (the carbon above the ring). The total number of glucose residues in a molecule of amylopectin is several thousand.
Starches are insoluble in water and thus can serve as storage depots of glucose. Plants convert excess glucose into starch for storage. The image shows starch grains (lightly stained with iodine) in the cells of the white potato. Rice, wheat, and corn (maize) are also major sources of starch in the human diet.
Before starches can enter (or leave) cells, they must be digested. The hydrolysis of starch is done by amylases.
With the aid of an amylase (such as pancreatic amylase), water molecules enter at the 1 -> 4 linkages, breaking the chain and eventually producing a mixture of glucose and maltose. A different amylase is needed to break the 1 -> 6 bonds of amylopectin.
Glycogen
Animals store excess glucose by polymerizing it to form glycogen. The structure of glycogen is similar to that of amylopectin, although the branches in glycogen tend to be shorter and more frequent. Glycogen is broken back down into glucose when energy is needed (a process called glycogenolysis).
In glycogenolysis,
- Phosphate groups — not water — break the 1 -> 4 linkages
- The phosphate group must then be removed so that glucose can leave the cell.
The liver and skeletal muscle are major depots of glycogen.
There is some evidence that intense exercise and a high-carbohydrate diet (“carbo-loading”) can increase the reserves of glycogen in the muscles and thus may help marathoners work their muscles somewhat longer and harder than otherwise. But for most of us, carbo loading leads to increased deposits of fat.
Cellulose
Cellulose is probably the single most abundant organic molecule in the biosphere. It is the major structural material of which plants are made. Wood is largely cellulose while cotton and paper are almost pure cellulose.
Like starch, cellulose is a polysaccharide with glucose as its monomer. However, cellulose differs profoundly from starch in its properties.
- Because of the orientation of the glycosidic bonds linking the glucose residues, the rings of glucose are arranged in a flip-flop manner. This produces a long, straight, rigid molecule.
- There are no side chains in cellulose as there are in starch. The absence of side chains allows these linear molecules to lie close together.
- Because of the many -OH groups, as well as the oxygen atom in the ring, there are many opportunities for hydrogen bonds to form between adjacent chains.
The result is a series of stiff, elongated fibrils — the perfect material for building the cell walls of plants.
Below are two videos of Mr.Matthew’s concise lectures on Carbohydrates. Enjoy!
Carbohydrates Word Search Puzzle
Carbohydrates
| S | J | H | R | G | L | U | C | O | S | E | L | A | G | R |
| A | E | D | I | S | A | C | C | H | A | R | I | D | E | S |
| R | L | D | L | E | Q | C | U | P | C | X | G | W | S | J |
| L | O | L | I | D | T | O | I | I | E | L | F | F | Z | S |
| Y | W | S | M | R | X | A | L | R | U | Y | N | J | R | X |
| X | B | W | R | G | A | Y | G | C | E | N | R | E | V | G |
| O | D | B | W | U | X | H | A | U | C | M | M | H | L | A |
| R | X | P | I | O | C | G | C | S | J | Y | O | Y | J | L |
| D | M | P | B | J | O | E | E | C | L | N | C | N | I | D |
| Y | M | R | U | N | Y | U | R | O | A | O | O | N | A | E |
| H | A | R | R | Z | D | G | P | P | S | S | S | C | G | H |
| C | R | H | A | I | M | T | A | I | C | U | Y | N | A | Y |
| F | Q | T | S | W | B | D | D | Q | L | R | W | L | Q | D |
| O | P | E | E | P | H | I | B | I | F | J | I | Z | O | E |
| N | R | O | V | O | C | S | N | E | L | H | V | I | V | P |
| ALDEHYDE | ANOMERIC | CARBOXYLIC |
| CONJUGATE | DISACCHARIDES | GLUCAGON |
| GLUCOSE | GLYCOSIDIC | HYDROXYL |
| INSULIN | POLYMERS | POLYSACCHARIDES |
| PRECURSOR | RESIDUES |
Quiz #1-The Cell
We were first tested on Wednesday 30th January on the Cell in groups of 5. The test was composed of 10 Multiple Choice questions which were fairly easy to answer. My group however received 9/10 even though we answered all of the questions correctly, to our knowledge. We still are not sure as to why we received this mark. Nevertheless, I enjoy partaking in these quizzes firstly because it’s easy marks to obtain and also because it allows me to keep on top of my work for I now expect a quiz on a weekly basis which encourages me to study even more!
Reflection on the cell.
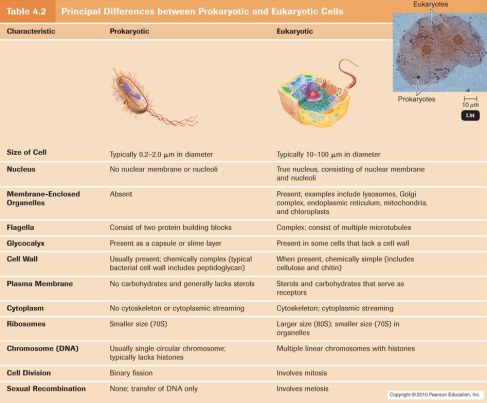
A cell may seem simple to define. However, valuable information can be left out in the process, rendering the explanation insufficient and incomplete. It can be defined as “The smallest unit capable of performing life functions.” This definition is too basic. What are these life functions? They include growth, metabolism, response to stimuli and replication. With immense help from Mr.Matthew’s YouTube videos, I was able to understand and correctly describe the structure and function of many organelles such as the cytoskeleton, lysosomes, proteasomes and ribosomes to name a few.
During this revision, I realized that I had forgotten some of the organelles’ functions, specifically the Endoplasmic Reticulum, which happens to be one of my favorite words in the English language. I now recall that the ER is a network of membranous tubules within the cytoplasm of a eukaryotic cell, continuous with the nuclear membrane. It usually has ribosomes attached and is involved in protein and lipid synthesis via the rough endoplasmic reticulum.
Mr. Matthew’s videos and lectures also encouraged me to do further research on sub-topics which I would not have on my own. One such example is with respect to Ribosomes. In prokaryotic cells, ribosomes are 70 s whereas in Eukaryotic cells, ribosomes are 80 s. But what does the “s” stand for? I have never questioned this before my lecture on Wednesday 23rd January, a fault of my own. Nevertheless, the “s” refers to svedberg unit (symbol S, sometimes Sv). Which is a non-SI unit for sedimentation rate. The sedimentation rate is the rate at which particles of a given size and shape travel to the bottom of the tube under centrifugal force.This reflects the rate at which a molecule sediments under the centrifugal force of a centrifuge.The svedberg is technically a measure of time, and is defined as exactly 10−13 seconds (100 fs)
[For some of you who did not heed Mr. Matthew’s gesture to research this]
In this lecture, we were also taught the differences between a Eukaryotic and a Prokaryotic cell, as well as the differences and similarities among a plant and animal cell. I have attached images below to illustrate these.
Introduction-My beginning with Biochemistry.
Hello everyone, my name is Candice Seenath. I am currently studying Biology as my major at The University of the West Indies, Trinidad in hope of pursuing my degree in Biotechnology later on. I however intend to pursue Biochemistry in the future to fulfill my dream of becoming a Forensic Scientist. My interests vary from Fashion to Football both of which I enjoy during my leisure time. My happiness stems from spending time with my family and friends but I also try to maintain a healthy balance between my social and academic life.
My first Biochemistry lecture officially began on the 23rd January 2013 with “The Cell”, a familiar topic. However, as Mr. Matthew aka Mr. BiochemJM explained, the caliber of work to be covered would be a lot more than was covered at the CAPE level and we would have to work extremely hard and diligently to achieve a good grade in his class. This was not an option but an order. My lectures in the first week went very smoothly to my surprise. I grasped the concepts and information well especially with the help of Mr. Matthew’s online lectures on YouTube . I look forward to my following classes!