A bond is said to be polar when there is a large amount of electronegativity difference between the atoms in a bond. Partial negative charges are found on the most electronegative atoms and the others are deemed partially positive.
Here are a few tips to aid in identifying the polarity and charges of amino acids.
1) If the amino acid side chain is a hydrocarbon, the amino acid is non-polar. There are 2 exceptions to this: Proline and Tryptophan. These exceptions however can easily be seen due to their steric hindrance.
2) If the amino acid chain has an atom that possesses electronegativity or polarity, but no charge, then the amino acid is polar uncharged.
3) If the amino acid has a charge on the side chain, it is polar.
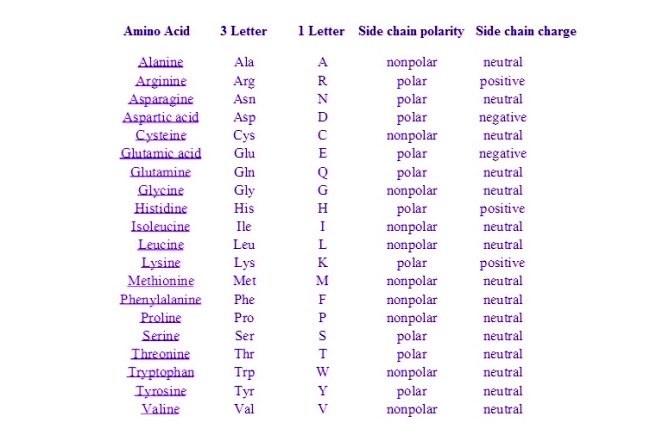
Below is a table of standard amino acid abbreviations and their side chain properties.