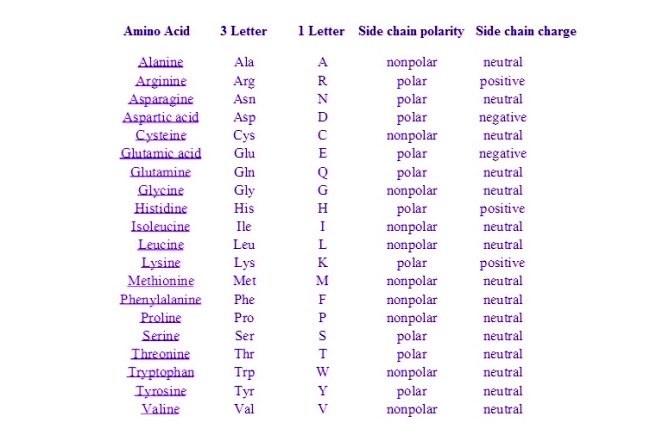
Amino acids differ when they have different R Groups attached to them. Learning the structures is important, but understanding why these molecules are arranged and placed where they are is equally important when learning and explaining this topic.
These groupings can be divided into :
1) Non-polar, aliphatic R groups.
Aliphatic R groups are nonpolar and hydrophobic. Hydrophobicity improves with increasing number of C atoms in the hydrocarbon chain. While these amino acids prefer to remain within protein molecules, alanine and glycine are ambivalent. This means that they can be inside or outside the protein molecule. Glycine has such a small side chain that it does not have much effect on the hydrophobic interactions.
2)Polar uncharged R groups.
There are eight amino acids with polar, uncharged side chains. Serine and threonine contain hydroxyl groups. Asparagine and glutamine contain amide groups. Histidine and tryptophan contain heterocyclic aromatic amine side chains. Cysteine has a sulfhydryl group. Tyrosine has a phenolic side chain. The sulfhydryl group of cysteine, phenolic hydroxyl group of tyrosine, and imidazole group of histidine all show some degree of pH-dependent ionization.
3) Aromatic R groups.
This group is identified by having an unsaturated ring containing double bonds in a conjugated form, especially containing a benzene ring. Examples of Aromatic R groups are : Phenylalanine, Tyrosine and Tryptophan.
4)Positively charged R groups.
In a neutral solution, the R group of a basic amino acid may gain a proton and become positively charged. Interaction between positive and negative R groups may form a salt bridge, which is an important stabilizing force in proteins.
5) Negatively charged R groups.
In a neutral solution, the R group of an acidic amino acid may lose a proton and become negatively charged.including aspartic acid (aspartate) and glutamatic acid (glutamate). In a neutral solution, the R group of an acidic amino acid may lose a proton and become negatively charged.
6)Sulfydryl groups.
These R groups contain a sulfur atom (S). The disulfide bond formed between two cysteine residues provides a strong force for stabilizing the globular structure.
For the structures and additional information on Amino acid groups, refer to the Amino acid and proteins part 1 lecture linked below.