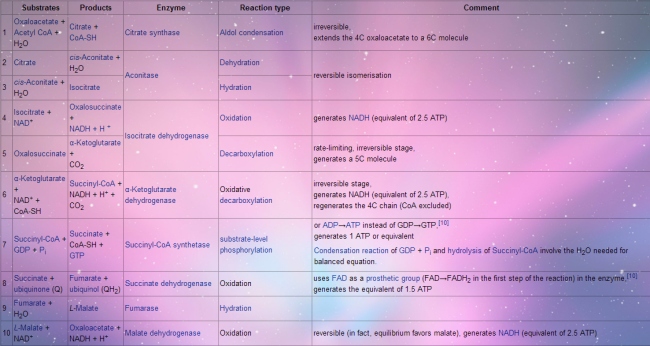
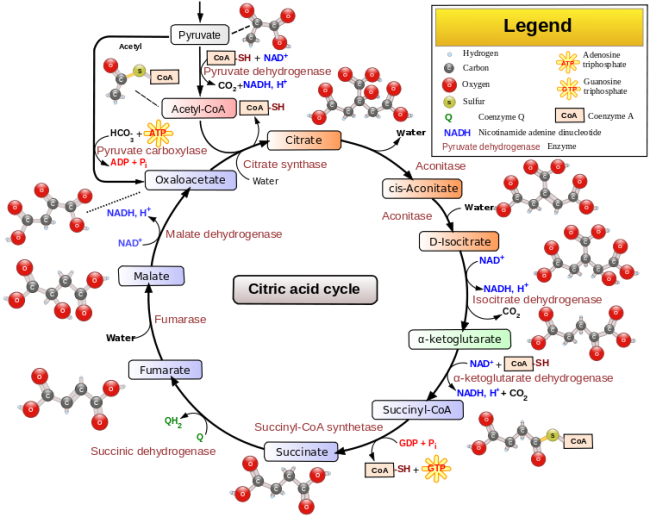
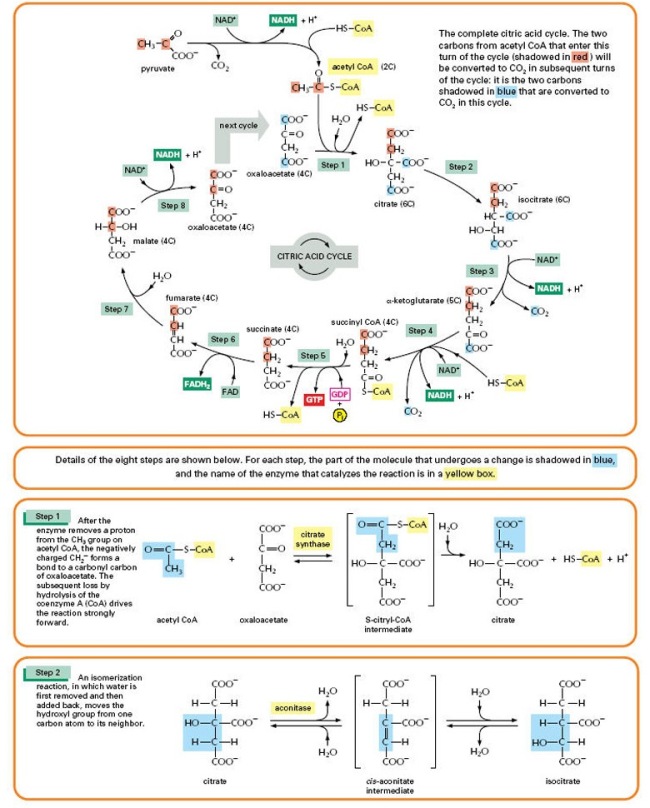
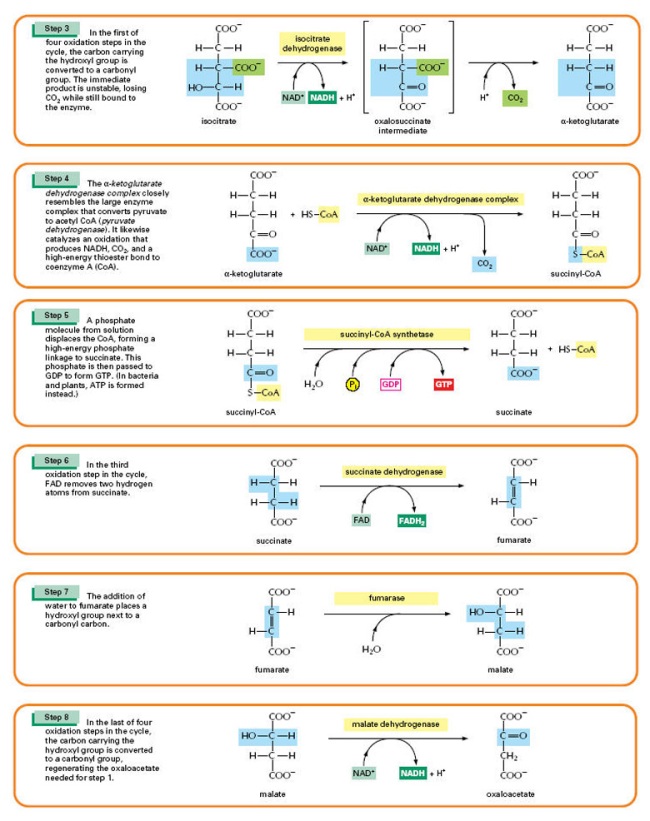
Video link: Crashcourse 2012. “DNA Structure and Replication: Crash Course Biology #10.” http://www.youtube.com/watch?v=8kK2zwjRV0M
Firstly, in the video, he speaks of DNA and what it encompasses in a brief point form manner. He states that nucleic acids are polymers, each one consists of many small repeating units. In DNA, they are referred to as Nucleotides; linked together they form polynucleotides. Next, he explains what a DNA molecule is made of (5-Carbon Sugar Molecule, Phosphate Group and 1 of 4 Nitrogen Bases). Of course you already knew this! The double helix is pair of molecules held tightly together, like a ladder. The sugar molecules & phosphates bind together thus forming the sugar-phosphate backbone at each side of helix, in opposite directions.
He continues to explain the concept of Directionality in a nucleic acid; the 5′-end and the 3′-end and vice-versa. This is explained in detail in the slides provided on MyElearning. The nitogenous bases can only be bonded to it’s specific partner, if you will. Adenine to Tryosine and Guanine to Cytosine, the base pairs. This concept is of great importantance in base sequencing. Next he speaks about RNA and how it differs to a DNA molecule. One such difference lies in its base pairs. Replication, as he speaks about next, is done by the “unzipping” of the double helix by the enzyme Helicase. You should be able to identify the “replication fork”, “leading strand” and the “lagging strand” on a basic diagram. These unwound sections are used as newly created templates, which will be used to create 2 complementary DNA strands leading in opposite directions.
DNA polymerase, adds matching nucleotides onto the main stem all the way down the molecule. The DNA polymerase is assisted in the beginning by RNA Primase, as it is only needed then. The DNA polymerase then follows suit and adds the rest of the bases to the leading strand. For the lagging strand, the process is slightly different. The DNA Polymerase can only copy strands in the 5′-3′ direction. The lagging strand however, is 3′-5′. DNA Polymerase therefore can only add new nucleotides to the 3 3′ end. RNA Primase, again enters at the beginning, the DNA Polymerase then enters moving backwards on the strand. DNA ligase join these fragments together, adhering the bonds to one another.
Throughtout the video he makes quirky remarks and comments as well as gives us fun and interesting facts through his “Biolo-graphy” session. Who knew that there were 6 Billion base pairs in EVERY cell! I certainly didn’t! Also, he gave a spontaneous pop quiz after the first 5 minutes of his lecture, a great way to maintain our focus! I was able to retain the information he explained, as I hope you will too!